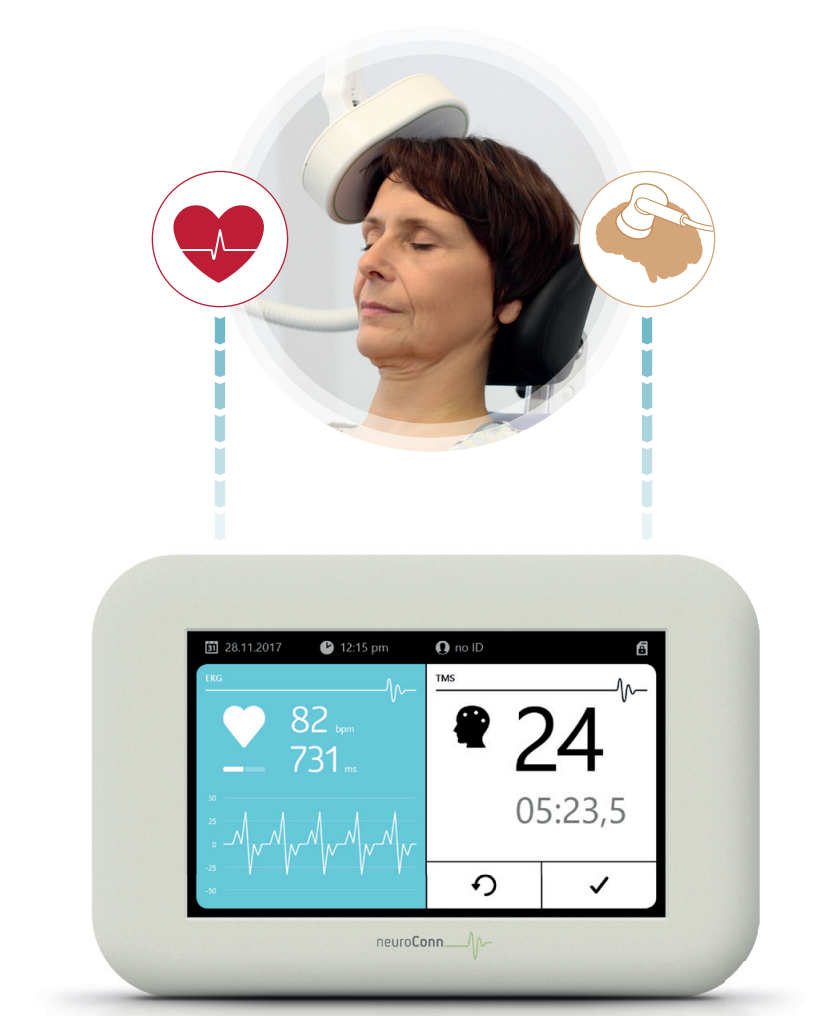
NCG-ENGAGE HR* is a biofeedback device according to the FDA Regulation Number 882.5050. The device allows the researcher to measure the heart rate during TMS of the DLPFC (dorsolateral prefrontal cortex). The primary objective of the NCG-ENGAGE HR* device is to systematically gauge and document heart rate metrics.
Easy-to-use ECG cable for safe recording during TMS
Touch screen to show data and user interaction
Stimulation output ECG stored on SD card (EDF+ format)
Connecting cables for receiving the trigger from different TMS devices

Items marked with * are investigational devices and for research use only. CAUTION - Investigational Device. Limited by Federal (or United States) law to investigational use.