Copyright & Disclaimer:
The 3D visualization is a simple representation on how neuroscience researchers integrate their neuromodulation devices in their research laboratory. The image presentation is for reference used only. Duplication, processing, distribution, manipulation, or any form of commercialization of such material shall require prior written consent. For information about the reproduction rights for any of the images contained within this site, please contact Jali Medical. Use of the presentation constitutes acceptance with the above copyright notice and all terms and conditions presented here.
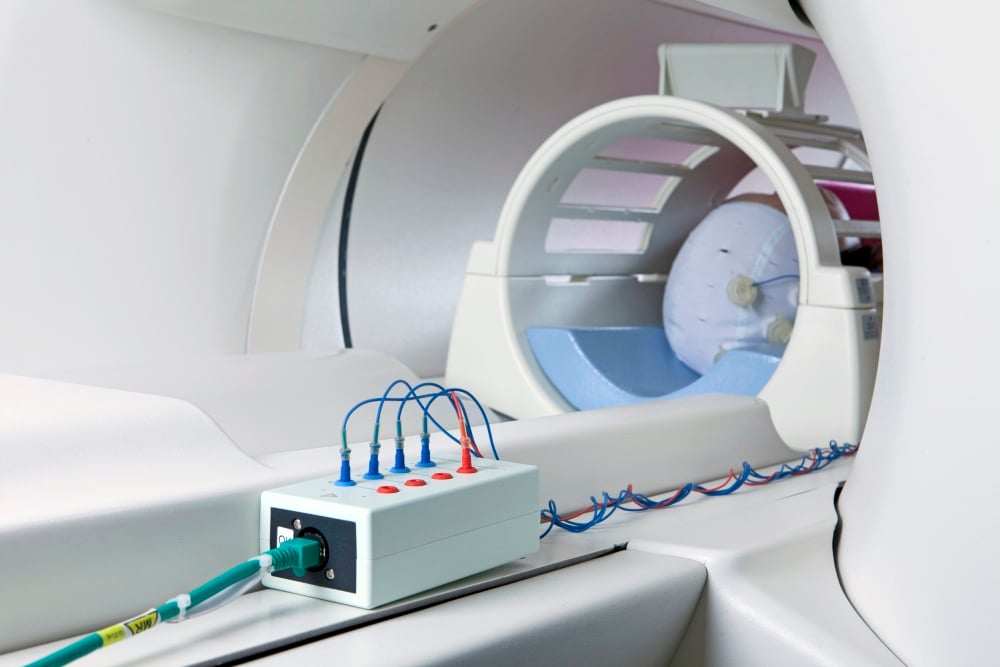
Closed-loop TMS-EEG integrates real-time EEG measurements into the TMS stimulation protocol to combine these two techniques. It permits the modification of TMS parameters based on ongoing brain activity, producing a feedback loop between stimulation and brain activity. It has several advantages over conventional TMS techniques. By integrating EEG measurements in real-time, TMS interventions can be precisely timed and targeted based on the dynamic brain activity. This individualized approach may improve the efficacy and specificity of TMS interventions used in various areas of neuroscience research, such as studying brain connectivity, investigating neural oscillations, exploring neuroplasticity mechanisms, and researching brain disorders. It has the potential to advance our understanding of brain function and open new therapeutic avenues for neurological and psychiatric disorders.
The term circuit derives from the Latin word circuitus, which means "to go around," implying a process or structure that returns to where it started after cycling. Technological advancements have significantly widened our understanding of electronic circuits, which are structures that constantly loop providing information and executing binary codes and specific tasks. Although thousands of times more complex, the brain follows a similar work cycle. Neuronal network circuits initiate activities in specific areas of the brain. Take the movement of an arm to reach an object as an example. The prefrontal cortex would plan the specific movement, then send information to the primary motor cortex, then to the spinal cord, and the muscles to execute the movement. The loop is closed when the sensory system sends information through the sensory pathway to tell the brain that the task was successfully completed. The complexity of the human nervous system vastly exceeds that of the most complicated set of integrated microchips, and the execution of complex tasks necessitates numerous coordinated processes occurring concurrently. This astounding complexity has driven us to investigate neural processes in systems in order to better understand their components and physiology. That perspective has considerably advanced our understanding of numerous processes. Motor control research institutions often set up "open-loop" protocols to artificially stimulate the nervous system and analyze the resulting motor output.
In the last 30 years, artificial brain stimulation has led to extensive development of knowledge of how the brain controls movement and behaves to execute actions; due to its features as non-invasive and painless, TMS has become key in this process, and extensive protocols have been used to examine the behavior of the motor cortex while participants execute specific motor tasks, receive sensory cues, and react to stimuli. Cortical excitability is influenced by many variables; "It is a field plagued with ill-understood intra- and inter-individual variability, effects that hold on average but not reliably for any one individual subject or patient" (Zrenner et al., 2016). EEG can provide a non-invasive, instantaneous view of cortical processes, and cortical waveform interpretation is becoming more comprehensive and accurate. New closed-loop paradigms use EEG specific averaged events that indicate brain states and can be utilized to predictably trigger TMS altering the output relevant to a given task.
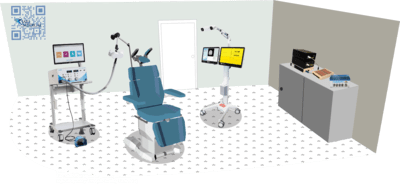
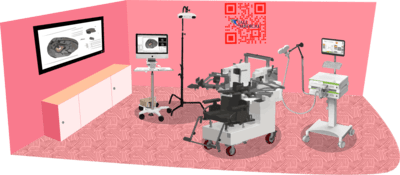
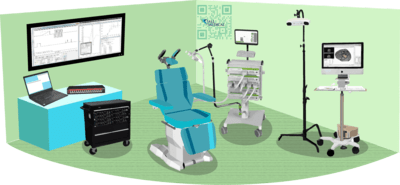
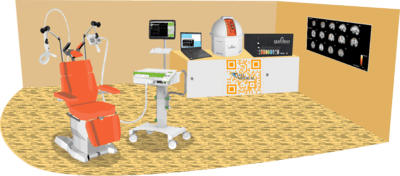
The level of complexity required for conducting this kind of research necessitates the use of several well-coordinated devices.

A MAG&More PowerMAG EEG ppTMS with numerous coil shapes possibilities, the most common being a figure-of-eight coil (Double coil PMD70-pCool) or a double-cone coil (Cone Shaped Coil) with a biphasic current waveform. Experimental studies involve sessions carried out with sham coils (defocalized technology). TMS is administered at the optimal cranium position to activate the targeted muscles. While there is no decrease in pulse power during stimulation over time, the biphasic waveforms can be delivered at full power output (100% intensity) up to 30 Hz with full external controllability via known systems like CED 1401 with Signal software, (Cambridge Electronic Design, Cambridge, UK) Matlab, or other programming platforms. With the the front-panel and external control, you may freely set every single pulse (shape, direction, and intensity), and continuous EEG can be recorded without any power line noise or recharging aberrations, enhancing the PowerMag Lab EEG stimulator series compatibility.
See Product Page
Active or passive electrodes, such as typical Ag-AgCl, can be used to record high resolution EEG. Setups can range from 40 to 160 channels. To reduce electromagnetic artifacts from TMS or MRI systems, the signal is amplified, filtered, and sampled. The recordings are captured with a wideband DC-3500 Hz measuring mode switchable channel by channel. The Windows 64-bit software supports online averaging and TMS artifact removal with 24 bit resolution and sampling rates up to 80 000 Hz per channel. Closed-loop TMS, for example, uses a 16-channel analog out option with a very low latency of 2.5 ms; an optional Real-time Digital Out is also available. Bittium NeurOne transmits a constant-phase trigger pulse to the EEG sampling rate, enabling accurate reproduction of the TMS-induced artifact and easy artifact removal.
See Product Page
State-dependent electroencephalography-triggered transcranial magnetic stimulation (EEG-TMS) can be used to target the peaks and troughs of the EEG traces, EEG online predicting analysis can be extracted from any part of the brain being monitored, and the different waveforms are separated by frequency and correspond to specific brain states (e.g. Gamma, Betha, Alpha, etc). Zrenner et al reported predictions based on the sensorimotor μ-rhythm in healthy subjects using a millisecond resolution real-time digital signal processing system. The authors of the research assessed corticospinal excitability by measuring the amplitude of motor evoked potentials in hand muscles.
See Product Page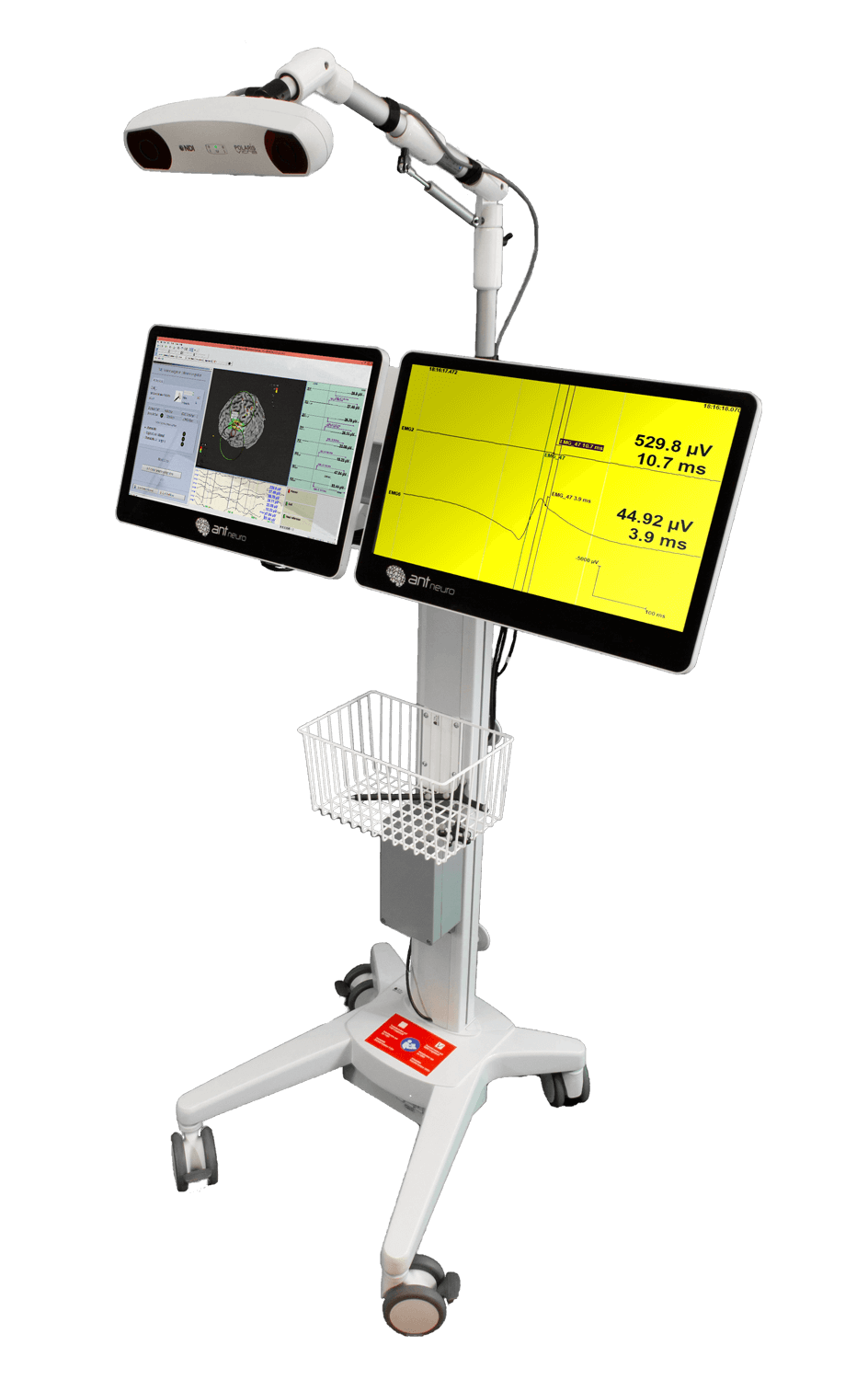
Neuronavigation data can be acquired precisely in real-time, personalized MRI DICOM files can be loaded into the resource library, or MRI-averaged dummy head models can be used to assign 3D coordinate markers. Simultaneous EEG-TMS recording is fully compatible with other systems, allowing for simultaneous recording and stimulation position recording. The premium version includes an 8-channel EMG as an add-on. To analyze motor maps online, motor mapping operations are incorporated as toolbars and color-coded MEP amplitudes are visualized in full 3D. Colored DICOM export of mapped functional hotspots can be done for further evaluation and processing.
See Product Page
• MAG&More´s “Air Cooling-Technology” reduces heating and allows for an increased number of stimulation pulses
• Specially designed for long protocols or high patient throughput
• Performance: more than 20.000 pulses at 1 Hz, 75%, 20°C start temp
• Weight: 2.6 kg
• No pulse counter which forces you to purchase a new coil after a certain amount of pulses

Individuals can be tested at random in the Edge-M TMS testing/subject chair, which allows for easy resetting of the head, decreasing time and variability between sessions with the same individuals and standardizing testing to reduce inter-individual variability. Edge M is totally adaptable and can be utilize to a wide range of protocol requirements, such as hand testing boards with force sensors or foot pedals with varying angles to change the position of joints during the test. In addition, the chair had customizable one or two heavy-duty coil holders with high weight capacity and extensive degrees of freedom controlled by a single knob. The head is supported comfortably while being securely stabilized to maintain testing locations.
See Product PageTranscranial direct current stimulation (tDCS) is a technique that uses low-amplitude direct electrical current delivered through scalp electrodes to modulate cortical activity. Anodal tDCS increases cortical excitability, while cathodal tDCS reduces it after a single session that typically lasts 20 to 30 minutes. The mechanism of action for tDCS likely relies more on modulation of ongoing neuronal activity than the induction of new neuronal activity since the electrical currents are insufficient to generate action potentials. tDCS has a favorable safety profile compared to other neurostimulation techniques, with only skin irritation at the electrode sites reported as side effects. In human studies, tDCS has been shown to modulate motor evoked potentials (MEP) in the motor cortex, with anodal stimulation increasing MEP amplitude and cathodal stimulation decreasing it. Longer duration of tDCS leads to longer-lasting after-effects, supporting the idea that repetitive sessions could lead to more stable and enduring neuroplasticity. However, a significant limitation of studies on non-motor cortical areas is the absence of a direct measure of cortical excitability, which is readily accessed in the motor cortex through MEP amplitude. To quantify cortical modulation in non-motor areas, such as the dorsolateral prefrontal cortex (DLPFC), scalp potentials can be measured with EEG or TMS-EEG can be used to directly analyze TMS-evoked cortical responses to stimulation of any cortical area. To coduct such studies, a clustered small tDCS electrode montage can be used to simulate the standard bipolar tDCS montage. For precise targeting of the left DLPFC, neuronavigation system should be employed with trackers that are placed on the subject's head and on the TMS coil for continuous online monitoring to ensure correct coil positioning throughout the experiment. It is possible to record TMS-evoked EEG potentials (TEPs) using an EEG system that is compatible with TMS to prevent EEG amplifier saturation and enabling data to be continuously recorded during TMS. This seamless setup orchestrated by CED for data acquisition and analysis, allows investigation of changes in cortical responses after interventional stimulation of the DLPFC using tDCS, which could lead to alterations in cortical activity and responsivity as measured by resting-state EEG and TMS-EEG.

The use of TMS in research requires an individual and precise positioning of the TMS coil at the selected brain region. MR-based neuronavigation systems can visualize the electromagnetic hot-spot of the coil in real-time at an individual, anatomical MR-data record. This enables the user to stimulate the target with great precision and makes reproducibility of the TMS set-up much easier.
Additional navigated TMS allows the researchers to protocol and map his work for studies and papers. Color coded fMRI data showing active regions of the brain is another helpful tool to find the right target areas.
See Product Page
The DC-Stimulator MR is an fMRI-compatible stimulator for use in scientific research. It is an extension of our single-channel DC-Stimulator PLUS, so it benefits from multi-stage monitoring and a complete range of tES modes of operation: transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS).
The DC-Stimulator MR allows the application of transcranial electrical stimulation (tES) during functional magnetic resonance imaging (fMRI) to localize the exact position of cortical activation.
See Product Page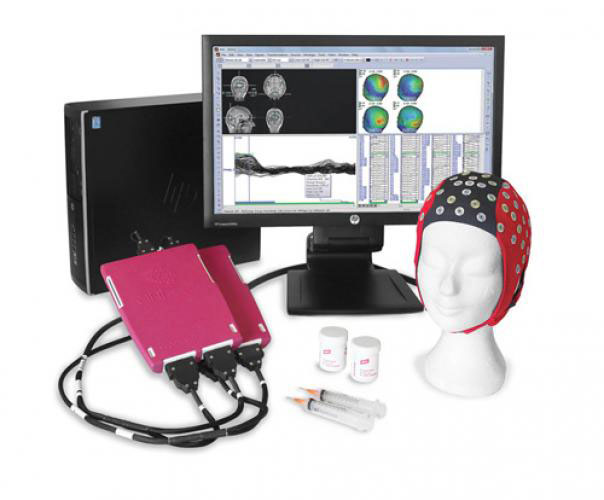
eegoSports has 24-bit resolution, an onboard battery with up to 5-hours of recording time, and weighing in at 500 grams, the compact eego amplifier is designed with mobility in mind. It offers the complete freedom to collect high-density EEG data, bipolar EMG signals, and a variety of physiological sensor data, wherever and whenever required, with publish quality data in less than 15 minutes! All recorded data are stored directly on the tablet which is carried in the backpack together with the amplifier. This aspect has a great advantage in prevention of data loss. As the amplifier and the tablet are connected directly with each other, the data transfer process does not depend on the quality of the wifi or Bluetooth connection and therefore the data are always safely stored on the tablet.
See Product Page

• 16 channels of 16-bit waveform input on base unit
• 1 Gbyte memory expandable to 2 Gbytes
• Dynamically programmable amplifier option
• Software-selectable ±5V or ±10V input and output ranges
• Synchronisation with other CED 1401s for large numbers of channels, all accurately timed
• USB 2.0 high-speed connection
• Firmware upgrades via CED web site
• Trigger input converter from high-level pulses or switch closures to 5V TTL
• Overload indicators with software readback
• EMG filter with full wave rectifier and programmable post-filter gain
• 16-bit ADC for digitisation of transducer and other low-bandwidth signals and transmission of results down the serial line

The increasing use of transcranial magnetic stimulation (TMS) in medicine requires individual and precise positioning of the TMS coil at the selected brain region.
The MAG&More PowerMAG View! 3D neuro-navigation system visualizes the electromagnetic hot-spot of the coil at an individual, anatomical data record. This enables the TMS system to stimulate the target with great precision. The PowerMAG View! combines functionality with simple handling. The result: Intuitive positioning with superb precision and reproducibility.
See Product Page
PowerMAG stimulation coils have been developed continuously together with our frequent users, resulting in coils with highest quality, accuracy and efficiency. By these means the very best outcome has been achieved, both in effectiveness and usability of the coils. Application-oriented coil geometries and the electromagnetic properties draw on all the potentials of the PowerMAG TMS system.
• Physiological efficiency: variety of stimulation coils for specific applications
• Maximum comfort: less coil heating and audible noise
• Precision: extraordinary temporal and spatial resolution
Hand function assessments are critical for evaluating and predicting the progression of motor control pathologies such as ALS, MS, SCI, Stroke, etc. The majority of these conditions exhibit symptoms such as weakness, lack of control, and poor coordination, making assessment challenging. Novel neuromodulation protocols intended for treating such conditions require a refined assessment and monitoring of the movement disorder, as well as assisted motion mechanisms that enable limited or weak movement that would otherwise be insufficient to perform traditional rehabilitation tasks.
Recent robotic technology enables people with acute and chronic impairments to move with assistance. In 2021, Keeling et al. demonstrated behavioral motor improvement in a pilot group of patients with subacute stroke using the bilateral Kinarm Exoskeleton. Dimyan MA, et al. 2022 have additionally studied the successful diagnostic and predictive value of robotic-assisted movement.
Several studies have looked into the benefits of combining approaches; early papers combining TMS-based plasticity protocols with robotic-assisted hand-grasping arm reaching highlighted the relevance of muscle targeting and action time coordination. Y. Lei et al., 2009. Novel rTMS protocols have shown intriguing variations between high frequencies, and iTBS protocols have been demonstrated to be superior to sham stimulation for enhancing treatment improvement observed in robot-assisted training. 2022, Zhang JJ et al.
In 2017, Lei Y, et al., demonstrated changes in participants who received rTMS; authors reported "enhanced generalization of visuomotor adaptation across the arms, which was not the case in the sham group". The findings confirm M1 involvement in use-dependent learning and imply that use-dependent learning can contribute not just to motor learning but also to motor generalization'.
The combination of robotic devices to assist mobility in motor-impaired populations and TMS, rTMS, and visual feedback has revealed a plethora of unique scientific problems.

The DuoMAG MP-Dual combines two DuoMAG MP units into a single stimulating coil to deliver fully programmable paired pulse stimulation. It is feasible to deliver accurate sub- and supra-threshold conditioning and test pulses by independently adjusting the pulse intervals and power level of each DuoMAG MP. This is beneficial for studying inter- and intra-cortical inhibition and facilitation. It is also feasible to connect two separate coils for other applications such as inter-hemispheric stimulation.
See Product Page
Neuronavigation enables researchers to precisely map and locate specific brain regions. It offers a comprehensive solution for brain imaging, data analysis, and navigation that enables users to identify and delineate specific target regions or structures within the brain, which results into the generation of a virtual 3D model of the patient's brain that can be viewed and manipulated within the software environment.
Integration of Neuronavigation with multiple imaging modalities, real-time tracking capabilities, and trajectory planning capabilities make it a valuable asset for neuroscientists.
See Product Page
Kinarm Exoskeleton Lab employs a complex linkage to enable horizontal arm movements in the plane of flexion and extension at the shoulder and elbow joints. Torque motors record the arm's motion in the horizontal plane and independently apply loads to each joint. The design provides control and feedback from the shoulder and elbow joints, allowing loads to be applied to the shoulder and/or elbow joints. Patterns of joint motion are recorded, and the system computes muscular torques. The hand is allowed to interact with environmental objects.
The Kinarm Exoskeleton Lab is ideally suited for subjects with stroke, spinal cord injury, or cerebral palsy due to the Exoskeleton's ability to support the subject's upper extremities against gravity.

DuoMAG coils feature stimulator controls for triggering and are ergonomically designed to ensure participant and user comfort. With such a wide range of applications, such as standard coils for thresholding, air and liquid cooled coils for longer protocols, placebo coils for blinded studies, and custom coils to suit your research application, such as delivering pairs of pulses to investigate inter-cortical inhibition or long trains of rTMS pulses to investigate membrane firing potential.
See Product PageTranscranial direct current stimulation (tDCS) is a noninvasive method of stimulating the brain using mild electrical currents applied to the scalp. Its purpose is to modulate cortical excitability and, as a result, influence behavior and brain function. However, our understanding of the neural mechanisms underlying the effects of tDCS on large-scale brain networks in humans is currently limited. To gain insight into this matter, it is possible to combine tDCS with functional brain imaging techniques such as functional magnetic resonance imaging (fMRI) or electroencephalography (EEG).
Functional MRI is a widely used neuroimaging technique for investigating the neural mechanisms involved in cognitive processes and motor functions. When combined with tDCS, it allows for the analysis of the neural mechanisms underlying the effects of tDCS, providing detailed spatial resolution across the entire brain. The integration of tDCS with MR imaging techniques offers a unique opportunity to directly manipulate neuronal function while monitoring the state of the brain. This integration enables researchers to study not only how tDCS modulates specific brain regions but also how it influences activity throughout the brain in terms of anatomical and functional connectivity. Moreover, this combination of techniques can provide valuable insights into the optimal parameters for stimulation, such as the specific brain areas, timing, and dosage. Recent studies utilizing this approach have identified changes in task-related brain activity at the stimulation site as well as in distant brain regions. One advantage of this combination is that it allows for the measurement of neuronal activity under the stimulating electrodes as well as in remote brain regions during electrical stimulation. However, it is important to note that the motor evoked potential (MEP) amplitude recorded from tDCS reflects changes in the transsynaptic excitability of large pyramidal neurons, whereas the blood oxygen level-dependent (BOLD) signal of fMRI represents the overall synaptic activity of all cortical neurons.
As discussed by Esmaeilpour and colleagues (2019), there are three primary types of studies that can be conducted using tDCS-MRI/EEG integration. The first type is montage selection studies, where functional imaging or EEG can aid in selecting the appropriate montage to target functionally active areas of the brain. The second type is prediction studies, where baseline fMRI or EEG measures can provide a baseline for neural network activation and help interpret later behavioral and neural responses to tDCS (known as "Baseline fMRI/EEG as Predictor"). Finally, mechanistic studies use fMRI/EEG to explore changes in the brain that underlie tDCS effects and to determine the specific areas, timing, and methods by which stimulation influences brain function and associated behavior. The integration of MRI/EEG with tDCS can be implemented through three different approaches: (1) Sequential-inside scanner approach, in which imaging/EEG data are collected before and/or after tDCS intervention inside the magnet bore, (2) Sequential-outside scanner approach, in which imaging/EEG data are collected before and/or after tDCS intervention with stimulation being performed outside the scanner, and (3) Concurrent approach, in which imaging data are acquired during (and often before and after) tDCS stimulation inside the scanner using MR-compatible tDCS and EEG electrodes. The sequential approach allows for the assessment of the functional brain state before stimulation and/or the enduring effects of stimulation, whereas the concurrent approach enables the study of the effects of stimulation on ongoing neural activity.

• 1 channel, unipolar (DC) and bipolar (AC) stimulation possible
• 4 standard modes - single (continuous stimulation) - pulse (cyclical stimulation activation/deactivation) - sinus (sinus wave) - noise (normally distributed)
• Frequencies adjustable up to 250 Hz, phase freely adjustable
• Adjustable current (DC) up to 4,000 µA in increments of 25 µA
• "Study mode" for blind processing of genuine and 'pseudo' stimulation (optional)
• External trigger input and output (optional)
• Up to 16-channel, bipolar (AC) stimulation
• Current strength and curve forms adjustable up to ±4,000 µA, AC current strength adjustable up to ±1,500 µA (peak-to-peak)
• Freely selectable application duration
• Frequencies adjustable up to 1,000 Hz, phase freely adjustable
• 4 standard modes – tDCS (continuous stimulation) – pulse (cyclical stimulation activation/deactivation) – sinus (sinus wave) – noise (normally distributed)
• Import and storage of any stimulation sequences
• MRI extension for use in (f)MRI
• Targeted, optimized high-definition (HD) stimulation

Bittium NeurOne system enables fully synchronized group measuring of up to 30 persons simultaneously, for example in different types of psychological studies. The solution is optimized for use with transcranial magnetic stimulators (TMS-EEG), with optional possibility to use it during magnetic resonance imaging procedures (fMRI-EEG). NeurOne Tesla EEG System offers more accuracy, cleaner signals, faster sampling, modular solutions and more flexibility. NeurOne uses a specially developed Tesla amplifier with built in features including high dynamic range and special reduction technology to remove magnetic artifacts for TMS-EEG and fMRI-EEG applications. The NeurOne EEG system is available in 40 to 160 channels consists of a Main Unit and 1 up to 4 Tesla 40 channel amplifiers.
See Product PageCortical motor neurons have evolved in humans to respond to the specific interactions we have with our environment. The cortex communicates through efferent pathways with the spinal cord and this communication requires multiple different motor neuron pools firing again and again relapsing each other constantly to maintain muscle fibers contracted. TMS is an artificial way to excite cortical neuron pools, a single-pulse applied nearby the homunculus can depolarize the cortex and many metrics of cortical function have been obtained using electromyography read the motor output e.g. conduction time, motor threshold, silent-period, recruitment curves, and motor mapping (Chen R., 2000).
Several authors have investigated the significance of multiple descending volleys in eliciting motor evoked potentials (MEPs), and the concept of temporal summation is crucial for evaluating motor function. In 2001, Deletis V. et al. reported recordings from the epidural space of descending volleys evoked in the cortex by both TMS and TES. The authors described direct (D) and indirect (I) waves in the epidural recordings, as many others had previously described the output of the motor cortex. (Terao Y, et at., 2000, Roth Y, et al., Di Lazzaro V, et al., 2003, Ni Z, et al. 2011). Since afferent output decreases following injury (e.g. SCI, MS, ALS, etc.), it is crucial to address spared conductivity that can be recovered, modulated, or trained in acute or chronic disease stages.
Paired pulse TMS has shown to be of tremendous use to understand the excitability of cortical neurons, several protocols have been described for intracortical facilitation (ICF), short interval intra-cortical facilitation (SICF) (To Kimura Et al.1996; Zieman Et al.1998b; Rothwell, 1999). More recent studies have looked into the value of SICI, LICI, and ICF as biomarkers to assess Alzheimer's disease and mild cognitive impairment (Mimura et. al, 2020), others have established the involvement of the motor cortex on Parkinson's disease and the potential value of pp-TMS as an evaluation tool on this condition (Saravanamuttu et. al, 2021).
Further research into expanding the concept of cortical biomarkers has been impeded by our growing knowledge of the cortical system and the technological complexities of this type of experiment. One of the earliest reports (Peterchev AV. et al., 2010) focused on the versatility of the controllable-pulse-parameter TMS (cTMS) system, in which controllable pulse durations are independent for each pulse, but not intensity.

DuoMAG MP-Quad allows users to combine the four stimulator pulses into a single pulse providing a pulse amplitude that equates to 140% of a single DuoMAG MP output. The DuoMAG MP-Quad can also be used with four separate coils to carryout interhemispheric (quadrilateral) stimulation.
"Regular rTMS (repetitive application of single pulses at a single frequency, e.g. 1 Hz or 20 Hz) is already known to show some long-term effects. In addition, theta burst stimulation (TBS) is already important but its effect are not that robust and sometimes variable. QPS was derived from studies that showed that repetitive paired-pulse stimulation could evoke some long-term effects. The concept of QPS was to increase the number of pulses to four.
See Product Page
TMS coil position in reference to head position and internal cortical structures are colocalized in a 3-dimensional cartesian space where digital coordinates are registered and saved and can be linked to multiple forms of data, such as MEPs or EEG recordings. After importing fMRI files into the resource library, the software creates a model of the brain that highlights the cortical active areas. The markers in colocalized MRI models that represent and match averaged brain to the participant can be allocated using MRI-averaged head models.
EEG electrode placements on the skull can be marked and linked to corresponding waveforms. Brainsight TMS also includes a 2-channel EMG, which allows for the integration of MEPs and TMS coil positions. This functionality allows for the programming of millimetric grids, which are ideal for cortical mapping research.
See Product Page

• 16 channels of 16-bit waveform input on base unit
• 1 Gbyte memory expandable to 2 Gbytes
• Dynamically programmable amplifier option
• Software-selectable ±5V or ±10V input and output ranges
• Synchronisation with other CED 1401s for large numbers of channels, all accurately timed
• USB 2.0 high-speed connection
• Firmware upgrades via CED web site
• Trigger input converter from high-level pulses or switch closures to 5V TTL
• Overload indicators with software readback
• EMG filter with full wave rectifier and programmable post-filter gain
• 16-bit ADC for digitisation of transducer and other low-bandwidth signals and transmission of results down the serial line

• Figure-8 butterfly coil with 2x 70mm windings.
• Suitable for focused cortical stimulation and rTMS applications.
• Two fans provide air cooling for prolonged use
• Anderson connector for compatibility across the DuoMAG range
• Variety of coils is available for your specific research needs
Since the first reports by Stefan et al. 2000, there has been further development in our knowledge of the spike timing dependent plasticity concept, stimulation can be applied coordinated to the motor area and the peripheral nerve at relatively high frequencies, e.g. 20Hz. "a comparison between PAS and rTMS as a control with measurements at 0 and 30 and 60 minutes with no significant difference in MEP potentiation between the new PAS protocol and the rTMS control, immediately after or after the first 30 minutes," Sathyan S. et al. 2021 reported. At 60 minutes after the new PAS, MEP potentiation remained considerably higher than after rTMS alone."
In 2021, Lei Y. Te al. described cerebellar dysfunction associated with cerebellar atrophy and decreased sensory feedback to the somatosensory cortex in patients with chronic SCI, stating that their findings "suggest that the reduced ability to learn from movement errors during reaching movements in humans with SCI involves abnormalities in the spinocerebellar structures."
Controlled tactile pressure given over time to digits in the hands of subjects being scanned with fMRI revealed adaptation to pressure over time. YG Chung et al., 2015. These neuroimaging studies have revealed new insights into useful protocols for testing proprioceptive pathways and cortical involvement in sensory information processing. Their results indicate that "cortical activity dynamically adapts to sustained pressure stimulation mediated by SA-I afferents, involving changes in the degrees of activation on cortical regions for tactile perception as well as inter-regional functional connectivity among them."
Testing proprioceptive pathways with tactile stimulation allows researchers to determine the neuromodulatory effects of experimental rehabilitation strategies such as PAS protocols that could target cortico-cortical pathways to evoke plasticity, and PAS protocols involving M1 motor synapses with cerebellar pathways involved in somatosensory movement learning.

The DuoMAG MP is a high-powered, easy-to-operate TMS device with the capacity to transmit high-energy pulses to a subject at a frequency of 2Hz, which is currently unmatched in the field. The DuoMAG capacitor, which is part of a highly mobile stimulator module, can emit pulses of up to 700J and may be put on a desktop or an adapted cart for easy maneuvering.
Because of its innovative modular design, the DuoMAG MP family of monophasic TMS systems may be customized to meet the evolving needs of individual labs. For paired pulse TMS applications, the DuoMAG MP stimulators can be merged into a single system (the DuoMAG MP-Dual), with the option to upgrade to the DuoMAG MP-Quad for innovative quadripulse stimulation.
See Product Page

A tactile stimulator with a pneumatic pressure mechanism is used to elicit a controlled proprioceptive response; tactile stimulation involves the activation of nerve receptors in the skin. The Galileo tactile stimulation system can elicit up to eight separate stimuli at the same time, which is known as pneumatic evoked response. The majority of the receptors are low-threshold, with fast conducting mechanoreceptive afferents. Stimulus response is derived from mechanoreceptor-rich areas of the skin, such as the hand and orofacial dermatomes.
See Product Page

• Figure of 8 V shaped ‘cone coil’ with two 120mm windings on a 100° angled surface
• T shape handle orientation
• Suitable for spinal stimulation and deep stimulation areas like Cerebellum
• Figure 8 butterfly coil with 2x 70mm windings
• Suitable for focused cortical stimulation and repetitive TMS applications
• Two fans provide air cooling for prolonged use in rTMS protocols and paired-pulse protocols.
Transcranial Magnetic Stimulation (TMS) is a non-invasive brain stimulation technique that uses magnetic fields to modulate neuronal activity in specific regions of the brain. Over the years, TMS has emerged as a promising, effective, and well-tolerated treatment option for individuals with treatment-resistant depression (TRD) who do not respond to conventional antidepressant medications. In 2008, the FDA granted approval for the use of TMS in the treatment of Major Depressive Disorder (MDD) in adults. However, significant advancements and refinements have taken place in TMS technology since its first use, leading to the development of new and emerging techniques that enhance and expand its clinical effectiveness. These advancements include the introduction of pulse train protocols such as theta burst stimulation (TBS) and high-frequency stimulation, as well as the utilization of neuronavigational systems and improved electromagnetic coil technology.
The standard depression protocol involves daily treatment sessions over a period of several weeks where TMS pulses are delivered to the left dorsolateral prefrontal cortex (DLPFC) that is responsible for mood dysfunction. The coil is positioned over the head and the stimulation site is located using conventional techniques such as using external anatomical landmarks until the desired response is generated, typically measured by a twitch in the patient's hand. On the other hand, neuronavigation can aid in the precise placement of TMS coil. This method, albeit successful and time-tested, lacks target engagement. On the other hand, neuronavigation utilizes advanced computer-assisted technology to precisely locate and target specific brain regions by using imaging data, such as MRI or CT scans, with real-time tracking and visualization tools. By ensuring accurate and consistent placement of the TMS coil, neuronavigation increases the precision and reliability of treatment. Targeting the DLPFC with neuronavigation allows for more targeted stimulation, leading to enhanced therapeutic effects. Notably, neuronavigation systems do not introduce additional risks but rather enhance the safety and precision of TMS treatment by providing real-time guidance based on the patient's individual anatomy.
TMS is also a valuable tool in clinical research, enabling scientists and clinicians to investigate and understand various aspects of brain function and disorders. The several clinical applications of TMS include treatment of neuropsychiatric disorders, mapping and modulating brain function, neuroplasticity and cognitive enhancement, and biomarker development. Concurrently, ongoing clinical trials continue to explore the potential benefits of TMS therapies for other psychiatric and neurological conditions, such as anxiety, schizophrenia, OCD, PTSD, and chronic pain. While these applications are not yet FDA-approved, they represent areas of active investigation and hold promise for future treatment options. Thus, by measuring and modulating brain activity, TMS has the potential to offer faster and longer-lasting outcomes than standard treatments, all with no to very minimal side-effects.

MAG&More TMS Therapy Systems are specifically designed for the safe and simple use of transcranial magnetic stimulation in therapeutic applications. The Apollo TMS Therapy System is meant to make TMS therapy simple and straightforward for the operator, and it achieves this through its unique, high-quality design, as well as the touchscreen user interface, which includes user-friendly assistance systems. The design is intended to provide the patient with an appealing, modern atmosphere.
TMS treatment has been approved by FDA since 2008 for patients with depression who have failed to achieve satisfactory improvement from one prior antidepressant medication at or above the minimal effective dose and duration in the current episode.
See Product Page
Features and benefits
• First FDA-cleared neuronavigation
*Complete, non-invasive neuronavigation solution for neuromodulation with TMS
• High precision MRI-guided e-field neuronavigation with real-time visualization of stimulated brain areas on a standard MRI or individual patient MRI imports
• Compatible with a broad range of TMS coils for each specific application
• Enables simultaneous tracking of up to two TMS coils
• Seamless EEG-EMG-TMS multimodality integration
• Replicable results and intuitive step-by-step workflow for the mapping of speech and motor functions
• Colored DICOM export of mapped functional hotspots for further use in surgical navigation systems

• Advanced hydrothermal cooling technology significantly reduces heating (performance 4.000 pulses at 1 Hz, 75%, 20°C start temp)
• Remarkably quiet and easy to handle, weight 1.4kg
• Same coil is used for motor threshold determination and subsequent therapy
• No pulse counter which forces you to purchase a new coil after a certain amount of pulses
The motor-cortex, the prefrontal cortex, and the somatosensory cortex, among other structures, are involved in the hand's motor function. Frequently, the interaction of multiple systems can be dissected on purpose, isolating hand function and even specific parts of hand function to very specific movements or tasks, such as index finger abduction to examine the activity of 1FDI. One advantage of such focalized testing is the diminution of variability among the results, the purity of the monosynaptic system represented in a small muscle that had a vast cortical representation, among others; this makes this and other hand muscles ideal for TMS testing paradigms; this testing requires specific hand/arm positioning and even blocking specific movements, and is performed in a seated position the majority of the time. While he learned a tremendous amount of new concepts from this particular setup, human hands move in a very complex manner in daily life, and we are capable of standing or even walking while using our hands unilaterally or bilaterally to achieve complex objectives. Few studies have examined the effects of testing hand/arm function while standing or walking, and the research infrastructure required to conduct such tests could be quite demanding. Stewart JC, et al., 2020, reported the significance of the concept. " Skilled movement of the arm and hand are important for the completion of everyday functional activities. While many functional tasks are performed in standing (e.g. meal preparation at the kitchen counter), most measurement of upper extremity function in clinical and research settings occurs in sitting. Research suggests that the performance of skilled arm and hand movements in standing differs from the same movements in sitting (Berrigan, Simoneau, Martin, & Teasdale, 2006; Cuisinier, Olivier, & Nougier, 2005), requires precise coordination between upper extremity movement and postural control (Chen, Lee, & Aruin, 2016; Pozzo, Stapley, & Papaxanthis, 2002), and is associated with changes in corticomotor excitability for upper extremity muscles (Kantak et al., 2013; Runnalls, Anson, & Byblow, 2017). The impact of standing on the control of upper extremity tasks may be magnified in individuals with neurologic conditions such as stroke who often present with postural control deficits (Belgen, Beninato, Sullivan, & Narielwalla, 2006; Harris, Eng, Marigold, Tokuno, & Louis, 2005; Jorgensen, Engstad, & Jacobsen, 2002; van de Port, Kwakkel, van Wijk, & Lindeman, 2006). Determining the effects of standing on upper extremity function effectively and efficiently, using a standardized outcome measure, could be useful to clinicians in understanding arm function in a manner that is better matched to how people move in real life and in determining if arm training activities in standing are warranted". Although the authors discovered differences, testing was primarily focused on manual dexterity tests, with limited information on the other systems involved. A setup designed for this purpose requires a robotic-assisted system that can sustain the upstanding position under controllable conditions, particularly for individuals with impaired standing.
The hand/arm testing can be analyzed under controlled paradigms and perturbation protocols can evaluate the motor system's ability to adapt and learn under specific conditions. The motor system can also be studied in conjunction with visual feedback testing protocols to acquire precise information about the focalization that the brain devotes to hand activity and the influence of other motor systems, subcortical and spinal. Multiple arm-leg muscles can be monitored simultaneously in a clean, non-obstructed wireless environment with the aid of novel wireless EMG systems, which can reduce protocol complexity.
The involvement of subcortical systems in relay spinal systems, such as maintaining balance in the standing position and while the upper limbs execute complex tasks, can be addressed by a combination of a vestibular sensor recording detailed information regarding changes in posture and balance. Understanding the capacity of the human central nervous system to effectively execute multiple combined tasks necessitates the collection of additional data from this multimodal setup.


The monitoring of the vestibular system requires a combination of sensors, accelerometers, and software. The PROTXX Phybrata sensor is an unobtrusive, non-invasive, wearable system that attaches behind the ear of a participant over the skin of the mastoid process. The Phybrata test requires the participant to simply stand still for a few seconds with their eyes open and closed. The tests can be extended, and the metrics can be customized for more extensive testing. The Phybrata sensor detects microscopic involuntary body motions, both normal motions characteristic of healthy individuals and pathological motions caused by physiological impairments that affect balance and postural stability.
See Product PageWhen high stiffness and hand-based haptic feedback are required for an experiment, the Kinarm End-Point Lab is the system of choice. Studies cover a wide range of topics, including eye-hand coordination, complicated cognitive challenges, human motor control, and learning in altered visual or mechanical contexts. Other neuromodulation devices, such as EMG, EEG, and TMS) that take digital or analog inputs/outputs can be integrated with Kinarm Labs. To ensure that the data is synchronized for both online use and post-experiment analysis, the Kinarm Lab may always obtain the data from the external systems in real-time.
See Product Page
The Trigno Wireless EMG System is a high-performing device designed to make EMG signal detection reliable and easy. Each EMG sensor has a built-in triaxial accelerometer, a transmission range of 40m and a rechargeable battery lasting a minimum of 7 hours. The system is capable of streaming data to EMGworks® Acquisition and Analysis software, and of generating 16 EMG and 48 accelerometer analog channels for integration with motion capture and other 3rd party data acquisition systems. Full triggering features further expand the possibility for integration with additional measurement technologies.
See Product PageErgoneers eye tracking glasses are accurate, slip-resistant, comfortable and adjustable which can be worn over prescription, sun, shutter, or polarized glasses. Research can be done in nearly any light. Vertical adjustable field and eye cameras allow natural head holding and optimal eye tracker accuracy, not to mention the modular design allows it to be used for a single sensor type, such as the D-Lab Eye Tracking Head Mounted, or in conjunction with a variety of other input channels, such as video or data stream.
D-Lab behavioral research data acquisition tool supports ergonomic and usability studies throughout their duration. It assists in study planning, data collection, and automated analysis. D-Lab can handle many participants, varied data channel frequencies, and simultaneous data recording.
See Product PagePreclinical research utilizing Transcranial Magnetic Stimulation (TMS) in animal models has proven instrumental in advancing our understanding of brain function and exploring potential therapeutic interventions. TMS studies in animals offer a multitude of applications across various research domains ranging from elucidating neural circuitry to exploring therapeutic interventions for neurological and psychiatric disorders. These translational findings contribute to the development of optimized TMS protocols and inform clinical applications in human patients.
Rodent models, such as mice and rats, have been extensively employed in preclinical TMS studies due to their well-established genetic tools, behavior assays, and relatively low cost. TMS in rodents offers unique opportunities to investigate neural plasticity, neuronal circuitry, and the effects of stimulation on behavior. By targeting specific brain regions with TMS, researchers can probe the causal relationship between neural activity and behavior, paving the way for understanding brain functions and pathologies. Studies utilizing TMS in rodents have revealed valuable insights into neurodevelopmental disorders, addiction, mood disorders, and cognitive impairments. For instance, repetitive TMS (rTMS) applied to the prefrontal cortex of rodents has demonstrated improvements in depressive-like behaviors and synaptic plasticity, mimicking the effects observed in human depression. Furthermore, mechanistic rodent studies have helped elucidate the molecular and cellular mechanisms underlying the effects of various TMS protocols that are currently used in the clinical arena, including changes in neurotrophic factors, neurotransmitter levels, and synaptic connectivity.
Primate models, such as macaques, have provided crucial translational insights into human brain function and disorders due to their highly analogous neuroanatomy and behavioral complexity. Preclinical TMS studies in non-human primates have proven instrumental in understanding the neural substrates underlying motor control, attention, executive functions, and decision-making. By combining TMS with advanced imaging techniques, researchers can investigate the network effects of TMS across interconnected brain regions in primates. This integration enables the mapping of functional connectivity, identification of effective targets, and exploration of TMS-induced changes in brain activity. Furthermore, primate models have facilitated the development of innovative TMS protocols, including theta burst stimulation, intermittent theta burst stimulation, and deep TMS, which have shown promising results for various neurological and psychiatric conditions.
Elicitation of motor evoked potentials (MEPs) in preclinical TMS studies requires precise timing and synchronization between the TMS pulses and EMG recordings. Specialized equipment, including trigger circuits and synchronization software, is employed to ensure accurate MEP measurements. Furthermore, appropriate filtering and amplification of the EMG signals are essential to obtain reliable and discernible MEP waveforms for analysis. To achieve accurate and targeted stimulation in preclinical TMS studies, the utilization of specialized coils is essential. Standard TMS coils used in clinical settings may not always be suitable for animal research due to the size and anatomical differences. Specialized coils designed specifically for small animal models, such as rodents and primates, allow for precise targeting of specific brain regions. These coils are often customizable and offer higher spatial resolution, enabling researchers to focus the magnetic field on smaller brain areas with greater accuracy. They can be optimized for various experimental requirements, including adjustable magnetic field intensity, focal point positioning, and temporal patterns of stimulation. Specialized coils also consider the specific anatomy and skull thickness of the animal species, facilitating efficient transmission of magnetic pulses to the desired brainregions.
The development and utilization of specialized coils in preclinical TMS studies ensure that the effects of stimulation are controlled, reproducible, and comparable across experiments.

The DuoMag MP is a single pulse mono-phasic stimulator that is primarily used in combination with EMG systems for MEP or collision studies. The high-energy pulse (700 Joules per pulse) of this stimulator is significantly higher than that of other stimulators that combine Bi-Phasic and Mono-Phasic pulses in one system. In the presence of motor and nervous disorders, the high-energy pulse is required for stimulating lower extremities and eliciting motor responses.
See Product Page
In addition to receiving triggers from external devices, the TruScan Research EEG can stream data in real-time via UDP for Brain-Computer Interface (BCI) applications, enabling investigations of how externally-triggered post-synaptic events evolve. The acquisition user interface is intuitively designed as a dashboard, and the review software permits simple yet potent analysis as well as expertise in a variety of standard file formats.
See Product Page
Jaltron coils are designs for use in human subjects (investigational devices*), non-human subjects, and in-vitro stimulation. Jaltron can manufacture stimulating coils that can be used with a variety of magnetic stimulators. A range of cooled stimulating coils, both linear and focal, designed for in-vitro stimulation of cell cultures. Available in low, medium, and high intensities with pulsed, cyclic, and static field output.See Product Page
Items marked with* are investigational devices and for research use only. CAUTION - Investigational Device. Limited by Federal (or United States) law to investigational use.